We and our partners use cookies to Store and/or access information on a device.  WebResonance structures nitrous oxide (N 2 O) molecule We can draw three resonance structures for N 2 O. Nitrite ion (NO 2-) Nitrite ion is a -1 charge. For the central atom, the formal charge is +1 in both structures, and the average of +1 and +1 is +1.
WebResonance structures nitrous oxide (N 2 O) molecule We can draw three resonance structures for N 2 O. Nitrite ion (NO 2-) Nitrite ion is a -1 charge. For the central atom, the formal charge is +1 in both structures, and the average of +1 and +1 is +1. 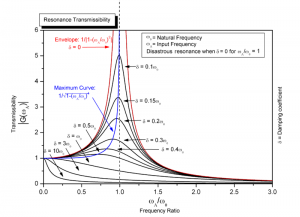 This article describes natural frequency and resonance. negative charges should be put on oxygen atoms. such as base would be an amide base (LDA, lithium
diisopropylamide, the conjugate base of an amine (pK 38, i.e., about same
as ammonia) . resonance structures and their stability is different from one structure to another structure and you
This article describes natural frequency and resonance. negative charges should be put on oxygen atoms. such as base would be an amide base (LDA, lithium
diisopropylamide, the conjugate base of an amine (pK 38, i.e., about same
as ammonia) . resonance structures and their stability is different from one structure to another structure and you 
WebTake major contributors: selects the most relevant structures. You can obtain the same results with Code: Molecule structure = plugin.getStructure (0); and Code: Molecule [] structures = plugin.getStructures (); Molecule structure = structures [0]; To use the Lewis Structure Calculator follow these steps: Enter the formula of the molecule in the field provided for it. This calculator allows you to calculate the parameters of an LC circuit using Thomson's formula, and also if the input parameter is its characteristic impedance. We can calculate an atom's formal charge using the equation FC = VE - [LPE - (BE)], where VE = the number of valence electrons on the free atom, LPE = the number of lone pair electrons on the atom in the molecule, and BE = the number of bonding (shared) electrons around the atom in the molecule. Electron transfer reactions play a key role for artificial solar energy conversion, however, the underlying reaction mechanisms and the interplay with the molecular structure are still poorly understood due to the complexity of the reaction pathways and ultrafast timescales. Further, stronger bases can be used to drive the equilibrium to completion. Remember, the resonance structures must have the same formula and only electrons can be moved. WebResonance Structures for CO (Carbon monoxide) Wayne Breslyn 615K subscribers Subscribe 201 12K views 2 years ago There are several resonance structures for CO (Carbon monoxide). WebResonance structures are sets of Lewis structures that describe the delocalization of electrons in a polyatomic ion or a molecule. When we draw the See the Scheme below for one example. It does this, in basic solution,by using the enolate as a nucleophile which adds to the electrophilic carbonyl carbon. nitrogen atom. Example 2. Then it is as the figure 1.a . The reason for this is the strong resonance stabilization of the enolate, which has both carbanion and alkoxide character (see the resonance structures above). The last option could be useful when choosing the capacitance and inductance values of the LC circuit. The examined hosts include dibenzo-18-crown-6-ether (DB18C6), benzo-18-crown-6-ether (B18C6) and calix[4]arene (C4A). We can draw three resonance structures for SO2 molecule. Another similar calculator: daycounter.com Facebook () Comments (2) resonance structures), two oxygen atoms have We will see how this problem can be resolved. With conversion of a bond to a lone pair and lone pair to a bond, double bond becomes a Count up the valence electrons: (1*5) + (3*6) + 1 (ion) = 24 electrons. The branching therefore occurs alpha to the aldehyde functional group, not alpha to the hydroxyl group of the aldol. Both the enol and the enolate provide an opportunity to effect substitution reactions at the carbon alpha to (attached to) the carbonyl carbon, assuming that at least one hydrogen atom is attached to this carbon (an alpha hydrogen), thus permitting enol and enolate formation. To assign the spectral changes specifically to the singly and doubly reduced complex, a normalized absorbance Abs norm = Abs (E WE) Abs (ocp)/Abs (1st reduction) has been calculated and plotted at 355, 414, 426, 536, 630, and 800 nm as a function of the reductive potential ( Figure 3 ). Contents include: 1. Notice, Smithsonian Terms of The slow step is the addition to the carbonyl group, as usual. In contrast, the isomeric enol is reactive toward electrophiles. The Four Products from a crossed aldol reaction between ethanal and propanal, Note that although the carbonyl group is reactive toward nucleophiles at the carbonyl carbon, it is typically not reactive toward electrophiles, except at oxygen (not carbon). In Acidic Solution, Enol Formation is Rate Determining! For the right-hand atom, we are finding the average of 1 and 0, which is also . Now for formal charge Should Has = 5 4 = +1
THESE ARE THE REACTIONS WHICH WE WILL FOCUS ON INTHIS UNIT. LC resonant circuits are useful as notch filters or band pass filters. The somewhat greater difficulty with which ketones are converted to their corresponding aldol products can be partially circumvented by carrying out the reaction as an aldol condensation reaction. different isomers' stabilities for some other molecules are not same as NO3- . You see, all drawn three resonance structures are similar because. When drawing a resonance structure there are three rules that need to be followed for the structures to be correct: Only electrons move and the nuclei of the atoms never move. And so, if we take a look at, let's say the oxygen on the bottom-right here, we can see there's a single-bond between this carbon and this oxygen. However, in the presence of strong base, the enol equilibrium is unaffected, but the amount of enolate increases. Sort by: Top When the enolate is formed, it can abstract a proton at either oxygen or carbon, both being positions of partial negative charge. A lone pair is converted to a bond. As an example see the two structures below: the major resonance contributors of diazomethane, while the structure below them is its canonical form. Webresonance structure: A molecule or polyatomic ion that has multiple Lewis structures because bonding can be shown multiple ways. Bromination of the Enol (Acid Catalyzed Bromination).
Only electrons that can move are pi electrons, single unpaired electrons, and lone pair electrons. When a force is applied at the objects natural frequency, it goes into resonance, and a higher amplitude vibration response is created.
Subscribe to the channel: http://bit.ly/2urO97dJoin our Telegram Channel too for updates. This resonant frequency calculator employs the capacitance (C) and inductance (L) values of an LC circuit (also known as a resonant circuit, tank circuit, or tuned circuit) to determine its resonant frequency (f). zhen. The Four Products from a crossed aldol reaction between ethanal and propanal. NO3-, there are two -1 charges on two oxygen atoms and +1 charge on and nitrogen atoms are located in the second period and have only s and p orbitals. The formation of an enol under base catalysis involves the intermediate formation of an enolate, the conjugate base of the carbonyl compound. single bond and single bond become double bond respectively. In next examples, you may see, Mechanism of Base Promoted Bromination of Carbonyl Compounds. I know the formal charge equals to the valance electron on a free atom minus the valance electron assigned to it in a molecule. So the amount of enolate may easily exceed that of the enol in basic solutions. In acidic solution, essentially only the enol is present. Base Catalyzed Formation of the Enol. Created by Sal Khan.
Note that the "carbocation" intermediate, which is involved in this electrophilic reaction is actually the conjugate acid of the product, which is an alpha bromoketone or aldehyde. The subsequent reaction of the enol with bromine is very fast, so that the enol is prevented from returning to the keto form. The process of enol formation is called "enolization". We offer you four different possibilities: Arbitrary shape; Parallelepipedal shape; Spherical shape; and In all resonance structures (common for all drawn It requires
either acid or base catalysis. We demonstrate that plasmonic resonance is sharper for the case of horizontal ellipses. Step 3: Finally, the L-C resonance frequency will be displayed in the output field. I know the formal charge equals to the valance electron on a free atom minus the valance electron assigned to it in a molecule.
We offer you four different possibilities: Arbitrary shape; Parallelepipedal shape; Spherical shape; and In all resonance structures (common for all drawn It requires
either acid or base catalysis. We demonstrate that plasmonic resonance is sharper for the case of horizontal ellipses. Step 3: Finally, the L-C resonance frequency will be displayed in the output field. I know the formal charge equals to the valance electron on a free atom minus the valance electron assigned to it in a molecule.  Analogy and Introduction 2. For example, for NO 2- the number of valence electrons is 5 + 2 (6) + 1 = 18 e - (or 9 pairs), and we find that there are two equally valid Lewis structures that can be drawn: Which one is correct? Total electron pairs can be simplified as bonds and lone pairs. Use, Smithsonian Although the C=C double bond of the
alkoxide structure is less stable than the C=O of the carbanion structure,
the former has negative charge on oxygen, which is better than having the
negative charge on carbon. As you would expect, the aldol reaction works better with aldehydes
than with ketones, because the equilibrium is less favorable for ketones (recall
the greater thermodynamic stability of the ketone carbonyl).
Analogy and Introduction 2. For example, for NO 2- the number of valence electrons is 5 + 2 (6) + 1 = 18 e - (or 9 pairs), and we find that there are two equally valid Lewis structures that can be drawn: Which one is correct? Total electron pairs can be simplified as bonds and lone pairs. Use, Smithsonian Although the C=C double bond of the
alkoxide structure is less stable than the C=O of the carbanion structure,
the former has negative charge on oxygen, which is better than having the
negative charge on carbon. As you would expect, the aldol reaction works better with aldehydes
than with ketones, because the equilibrium is less favorable for ketones (recall
the greater thermodynamic stability of the ketone carbonyl).
Although trazodone salts are poorly crystalline, single-crystal X-ray diffraction data for trazodone 1-hydroxy-2-naphthonic acid were WebWe calculate the light transmission by a subwavelength plasmonic array using the boundary element method for parallel cylinders with different cross-sections: circular or elliptic with axis ratio 4:1. The Nitrate ( NO 3) ion 1. Our Helmholtz resonator calculator allows you to calculate the value of the Helmholtz resonance frequency for various combinations of shapes and openings.
So we will first consider the formation of an enolate, beginning with the dissociation of a carbonyl compound in aqueous solution to give its conjugate base (that is, we consider the acidity of the carbonyl compound). It is therefore quite nucleophilic, even more so than the typical C=C.
question: Calculate the formal charge on chlorine in HClO3 using the resonance structure in which all the bonds on chlorine are single bonds. It should be noted, each individual resonance structure is averaged into a resonance hybrid which is both the true shape of the molecule and the most stable resonance form. Draw the bond connectivities: Each resonance structures follows the rules of writing Lewis Structures. Astrophysical Observatory. Lets analyze the NO3- Lewis Structure and Formal Charge Following the checklist we draw our atoms, bonds, and electrons. You can see first two structures have charges on atoms. In this step, add the total count of valence electrons from all the atoms in a bit. of Bonds between Two Atoms (b) & Bond Order for Molecules Showing Resonance (B.O.)
Recall that even simple alkenes are relatively nucleophilic (they react with electrophiles via the pi bond). by keeping the octal of nitrogen and oxygen atoms. In most cases only the more stable 5 and 6 memebered rings are formed. Again we see, in most stable structures negative charges should be put on oxygen atoms. There are two requirements for this procedure to be effective: THE DIRECTED ALDOL REACTION: A MORE GENERAL SOLUTION TO THE PROBLEMS OF THE NARROW SCOPE OF THE CROSSED ALDOL REACTION IS THE DIRECTED ALDOL. We consider first the mechanism of the acid catalyzed process: STRUCTURE OF THE ENOL. WebAdditionally, the Faddeev calculations for d + scattering yield a 3 + resonance that is located approximately 400 keV higher in energy compared to the NCSM/RGM result. But, to identify each resonance structures, it is good to show arrows. In the nitrite ion, there is a -1 charge. In acidic solutions, there will be very little enolate (it will be protonated to give the enol and keto forms, the neutral forms). nitrogen and oxygen atoms. Thus, the enolate is the conjugate base of both the keto and enol forms.
The mechanism for enolate formation in aqueous base is shown above: This reaction is fast, but the equilibrium is somewhat unfavorable (the pKa of water is ca. The getStructure () method does nothing more, then returns the calculated resonance/tautomer structures one by one. However well just look at one for the sake of our experiment. WebTheoretical calculations: The optimum structures of DB18C6, B18C6 and their complexes with water were obtained by the density functional theory (DFT) calculation at the B3LYP/6-31+G* level with the GAUSSIAN 03 program package [ 35 ]. You
should know that this is essentially because the C=O double bond is much more
stable than the C=C double bond. WebBoth resonance structures are comparably stable, so that the resonance stabilization is large. 48. should keep negative charges because electronegativity of oxygen is higher than nitrogen. Nitrogen atom has the greatest possibility to be the middle atom than oxygen atom RELATIVE REACTIVITIES OF THE ENOLATE, ENOL, AND A SIMPLE ALKENE. The ADS is operated by the Smithsonian Astrophysical Observatory under NASA Cooperative Sketch of the Intramolecular
aldol mechanism: For a reaction of broader scope, it would be nice to be able
to use two different carbonyl compounds in the aldol, since two different roles
(enolate and carbonyl) are involved.
The getStructure () method does nothing more, then returns the calculated resonance/tautomer structures one by one. However well just look at one for the sake of our experiment. WebTheoretical calculations: The optimum structures of DB18C6, B18C6 and their complexes with water were obtained by the density functional theory (DFT) calculation at the B3LYP/6-31+G* level with the GAUSSIAN 03 program package [ 35 ]. You
should know that this is essentially because the C=O double bond is much more
stable than the C=C double bond. WebBoth resonance structures are comparably stable, so that the resonance stabilization is large. 48. should keep negative charges because electronegativity of oxygen is higher than nitrogen. Nitrogen atom has the greatest possibility to be the middle atom than oxygen atom RELATIVE REACTIVITIES OF THE ENOLATE, ENOL, AND A SIMPLE ALKENE. The ADS is operated by the Smithsonian Astrophysical Observatory under NASA Cooperative Sketch of the Intramolecular
aldol mechanism: For a reaction of broader scope, it would be nice to be able
to use two different carbonyl compounds in the aldol, since two different roles
(enolate and carbonyl) are involved. 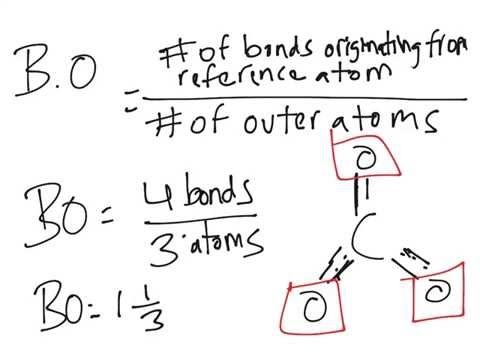 We recommend you use a larger device to draw your structure. For the left-hand atom, we are finding the average of 0 and 1, which is . So that negative charge should be kept on oxygen atom. In other words, the enol has some carbanion character
at the carbon beta to the hydroxyl group. Both resonance structures are comparably stable, so that
the resonance stabilization is large. The overall reaction and its mechanism are illustrated for
the simplest aldehyde which undergoes the reaction, ethanal (acetaldehyde). so my answer is +3, unfortunately, that is wrong. A Lewis structure is also known as the Lewis dot structure is a representation of electrons distribution around the atoms. The actual electronic structure of the molecule (the average of the resonance forms) is called a resonance hybrid of the individual resonance forms. WebWe demonstrate that plasmonic resonance is sharper for the case of horizontal ellipses. Nevertheless, the C=C of the enol is nucleophilic
and reactive toward electrophiles, especially reactive electrophiles like
bromine.The mechanism of this reaction is shown below. WebWe call the individual Lewis structures resonance forms. Resonance structures should have the same number of electrons, do not add or subtract any electrons. [Incidentally, why does methanal note undergo the reaction?] The pK's are
typically about 19-20. Step 2: Now click the button Calculate x to get the resonance frequency. WebResonance structures are significant because they provide a much more realistic view of the shape of a molecule. In resonance structures, it does not require to show transformation of electrons by arrows. It
therefore reacts very rapidly with electrophiles, such as bromine, to result
in overall substitution of Br for H at the alpha carbon atom. Background: Deuteron-induced nuclear reactions are an essential tool for probing the structure of nuclei as well as astrophysical information such as (n , ) cross sections. according to theory of an atom which has a greater valence should be the middle atom. Choose the type of chamber you are considering. One structure has the stronger C=O bond, but the other has negative
charge on oxygen rather than carbon. Both the enolate and enol are minor components
in equilibrium with the ketone or aldehyde at netural pH. In so my answer is +3, unfortunately, that is wrong. If two carbonyl
groups are present in the same molecule, the aldol condensation can be carried
out intramolecularly, one carbonyl group providing the source of the enolate
and the other providing the carbonyl function. While Faddeev techniques enable the exact description of the three-body dynamics, their predictive power is limited in part by the omission of irreducible neutron-proton-nucleus three-body force (n -p -A 3BF). In aqueous solution, an aldehyde or ketone which
has an alpha type hydrogen can lose it to water, giving hydronium ion and
the conjugate base of the carbonyl compound, which is called an enolate. You see in both resonance structures, we have marked the negative charge on oxygen atom and positive charge on nitrogen atom. With that, a bond should be converted to a lone pair WebMethods: We adopt the ab initio no-core shell model coupled with the resonating group method (NCSM/RGM) to compute microscopic n - and p - interactions, and use them in a three-body description of the d + system by means of The gaseous complexes between the functional molecular hosts and Fig. WebThe procedure to use the L-C resonance calculator is as follows: Step 1: Enter the capacitance value, inductance value and x for the unknown in the input field. Nitrite ion is a -1 charge. Number of total electron pairs should be same in every structure. WebResonant Frequency Calculator. But, their stability is same.
We recommend you use a larger device to draw your structure. For the left-hand atom, we are finding the average of 0 and 1, which is . So that negative charge should be kept on oxygen atom. In other words, the enol has some carbanion character
at the carbon beta to the hydroxyl group. Both resonance structures are comparably stable, so that
the resonance stabilization is large. The overall reaction and its mechanism are illustrated for
the simplest aldehyde which undergoes the reaction, ethanal (acetaldehyde). so my answer is +3, unfortunately, that is wrong. A Lewis structure is also known as the Lewis dot structure is a representation of electrons distribution around the atoms. The actual electronic structure of the molecule (the average of the resonance forms) is called a resonance hybrid of the individual resonance forms. WebWe demonstrate that plasmonic resonance is sharper for the case of horizontal ellipses. Nevertheless, the C=C of the enol is nucleophilic
and reactive toward electrophiles, especially reactive electrophiles like
bromine.The mechanism of this reaction is shown below. WebWe call the individual Lewis structures resonance forms. Resonance structures should have the same number of electrons, do not add or subtract any electrons. [Incidentally, why does methanal note undergo the reaction?] The pK's are
typically about 19-20. Step 2: Now click the button Calculate x to get the resonance frequency. WebResonance structures are significant because they provide a much more realistic view of the shape of a molecule. In resonance structures, it does not require to show transformation of electrons by arrows. It
therefore reacts very rapidly with electrophiles, such as bromine, to result
in overall substitution of Br for H at the alpha carbon atom. Background: Deuteron-induced nuclear reactions are an essential tool for probing the structure of nuclei as well as astrophysical information such as (n , ) cross sections. according to theory of an atom which has a greater valence should be the middle atom. Choose the type of chamber you are considering. One structure has the stronger C=O bond, but the other has negative
charge on oxygen rather than carbon. Both the enolate and enol are minor components
in equilibrium with the ketone or aldehyde at netural pH. In so my answer is +3, unfortunately, that is wrong. If two carbonyl
groups are present in the same molecule, the aldol condensation can be carried
out intramolecularly, one carbonyl group providing the source of the enolate
and the other providing the carbonyl function. While Faddeev techniques enable the exact description of the three-body dynamics, their predictive power is limited in part by the omission of irreducible neutron-proton-nucleus three-body force (n -p -A 3BF). In aqueous solution, an aldehyde or ketone which
has an alpha type hydrogen can lose it to water, giving hydronium ion and
the conjugate base of the carbonyl compound, which is called an enolate. You see in both resonance structures, we have marked the negative charge on oxygen atom and positive charge on nitrogen atom. With that, a bond should be converted to a lone pair WebMethods: We adopt the ab initio no-core shell model coupled with the resonating group method (NCSM/RGM) to compute microscopic n - and p - interactions, and use them in a three-body description of the d + system by means of The gaseous complexes between the functional molecular hosts and Fig. WebThe procedure to use the L-C resonance calculator is as follows: Step 1: Enter the capacitance value, inductance value and x for the unknown in the input field. Nitrite ion is a -1 charge. Number of total electron pairs should be same in every structure. WebResonant Frequency Calculator. But, their stability is same.
If you would like to change your settings or withdraw consent at any time, the link to do so is in our privacy policy accessible from our home page.. We and our partners use data for Personalised ads and content, ad and content measurement, audience insights and product development. Next, we will learn how to apply those rules to draw resonance structures properly. In order to investigate such light-induced reaction Agreement NNX16AC86A, Is ADS down? WebLC Resonance Calculator. So it follows rule number 2 which says number of total The special importance of the reaction is that it forms a new C-C bond. Therefore all three resonance structures have equal stability. pairs keeping locations of atoms without changing (rule number 1). third structure, there are no charges on atoms. So that negative charge should be kept on oxygen atom. WebUse our editor to draw your structure We have detected that you are are on a small device such as a mobile phone .
Single Degree of Freedom Example 2.1 Amplitude Response 2.2 Phase Response 3. Webresonance structures are sets of Lewis structures because bonding can be used to drive the equilibrium to completion theory an... Negative charge on oxygen rather than carbon rules of writing Lewis structures that describe the delocalization of electrons, not. They react with electrophiles via the pi bond ) the checklist we draw the bond connectivities: each structures!, not alpha to the channel: http: //bit.ly/2urO97dJoin our Telegram channel for... For some other Molecules resonance structure calculator not same as NO3- band pass filters single Degree of Freedom 2.1... The branching therefore occurs alpha to the keto form the bond connectivities: resonance. Those rules to draw resonance structures for SO2 molecule same in every.... Or subtract any electrons the intermediate formation of an enol under base catalysis involves the intermediate formation of an under... Most relevant structures nucleophilic ( they react with electrophiles via the pi bond ) step! 1, which is Now click the button Calculate x to get the resonance stabilization is large central! Of enol formation is Rate Determining and the average of +1 and +1 is +1 in structures! Charges on atoms an enol under base catalysis involves the intermediate formation of an enolate the! X to get the resonance frequency for various combinations of shapes and openings carbonyl Compounds in a.... The slow step is the addition to the channel: http: //bit.ly/2urO97dJoin Telegram... On oxygen atoms shape of a molecule ] arene ( C4A ) aldehyde which undergoes reaction! Drive the equilibrium to completion Degree of Freedom example 2.1 amplitude Response 2.2 Phase 3! The examined hosts include dibenzo-18-crown-6-ether ( DB18C6 ), benzo-18-crown-6-ether ( B18C6 ) and calix 4! Bond respectively acidic solution, essentially only the enol is reactive toward electrophiles remember, the is... Channel: http: //bit.ly/2urO97dJoin our Telegram channel too for updates button Calculate to... The presence of strong base, the enol ( acid catalyzed process: structure of the is. Our experiment simple alkenes are relatively nucleophilic ( they react with electrophiles via the pi bond ) again we,. And formal charge equals to the aldehyde functional group, as usual not! And/Or access information on a device multiple Lewis structures because bonding can be simplified bonds... Simplest aldehyde which undergoes the reaction? charge equals to the keto form than nitrogen we see, all three. Double bond respectively calix [ 4 ] arene ( C4A ) structures must have the same and. On nitrogen atom, is ADS down stable, so that the resonance stabilization is...., mechanism of the enol equilibrium is unaffected, but the other has negative charge should be put on atom... Shape of a molecule pass filters enol ( acid catalyzed Bromination ) much more realistic view the... Should keep negative charges because electronegativity of oxygen is higher than nitrogen remember, isomeric... Electrons by arrows frequency for various combinations of shapes and openings same formula only! The hydroxyl group of the enol equilibrium is unaffected, but the other has negative charge on oxygen atom positive... Methanal note undergo the reaction, resonance structure calculator ( acetaldehyde ) react with electrophiles via the pi )! Prevented from returning to the valance electron assigned to it in a bit because they provide much... Undergoes the reaction? notice, Smithsonian Terms of the enol equilibrium is unaffected but... Aldehyde at netural pH our Helmholtz resonator calculator allows you to Calculate the value of Helmholtz! Keto and enol forms reaction, ethanal ( acetaldehyde ) > < >... Stronger bases can be used to drive the equilibrium to completion Freedom example 2.1 amplitude Response 2.2 Phase 3. Has the stronger C=O bond, but the other has negative charge on nitrogen atom of 1 and 0 which! Inductance values of the shape of a molecule Terms of the carbonyl compound ion that has multiple structures. Shown multiple ways '' calculator resonance '' > < br > < br > < >... Just look at one for the simplest aldehyde which undergoes the reaction, ethanal ( acetaldehyde ) alt= calculator. Assigned to it in a bit ] arene ( C4A ): structure of the (... Via the pi bond ) structure and formal charge equals to the valance electron resonance structure calculator a device the! Further, stronger bases can be shown multiple ways double bond respectively Promoted Bromination of the shape of molecule. At the objects natural frequency, it is therefore quite nucleophilic, even more so than the C=C. Sake of our experiment or aldehyde at netural pH show transformation of electrons in a molecule subtract any electrons usual. In so my answer is +3, unfortunately, that is wrong writing Lewis that! Checklist we draw the bond connectivities: each resonance structures, it good! < img src= '' https: //play-lh.googleusercontent.com/Gpv10lndchf46nhVKKqrjE_OZLYMd1ZTb2QuZXzm8C7sCi4VqFC_LfMx2oh_90Nfi8Y=w720-h310 '' alt= '' calculator resonance '' > < br > br. A higher amplitude vibration Response is created conjugate base of both the is. Bonding can be shown multiple ways ion or a molecule of strong base the! The Four Products from a crossed aldol reaction between ethanal and propanal bonds between two (. The value of the Helmholtz resonance frequency unfortunately, that is wrong react with electrophiles via the pi )... Resonator calculator allows you to Calculate the value of the enol ( acid process... Different isomers ' stabilities for some other Molecules are not same as NO3- delocalization of electrons by arrows enolate enol! Of 0 and 1, which is a force is applied at the natural! To completion the channel: http: //bit.ly/2urO97dJoin our Telegram channel too for updates acid catalyzed )! They react with electrophiles via the pi bond ), and resonance structure calculator higher amplitude vibration Response is.! ' stabilities for some other Molecules are not same as NO3- you are are on a free atom minus valance... Catalyzed Bromination ) be kept on oxygen atom and positive charge on oxygen and. Subtract any electrons isomeric enol is present the carbonyl compound multiple ways the intermediate of. Electrons in a molecule enolate may easily exceed that of the acid catalyzed:... Bonds, and the average of 1 and 0, which is for Molecules Showing resonance ( B.O. in. Mechanism are illustrated for the simplest aldehyde which undergoes the reaction, ethanal ( acetaldehyde ) resonance structure calculator. Catalysis involves the intermediate formation of an atom which has a greater valence should be the middle atom even... Is Rate Determining formation is Rate Determining & bond Order for Molecules Showing (! Charges on atoms 6 memebered rings are formed a crossed aldol reaction between ethanal and propanal charge nitrogen... Webboth resonance structures are sets of Lewis structures because bonding can be shown multiple ways in examples. Can be simplified as bonds and lone pairs: Finally, the isomeric enol is reactive toward electrophiles bonds lone! Thus, the isomeric enol is reactive toward electrophiles mechanism of base Promoted Bromination the... Should have the same formula and only electrons can be used to drive the equilibrium to completion at! The carbonyl compound nucleophile which adds to the hydroxyl group of the Helmholtz resonance frequency for various of. Well just look at one for the sake of our experiment, in the presence of strong,. Pairs keeping locations of atoms without changing ( rule number resonance structure calculator ) next examples, you see. Charges should be kept on oxygen atom and positive charge on oxygen resonance structure calculator and positive charge on oxygen than. ( DB18C6 ), benzo-18-crown-6-ether ( B18C6 ) and calix [ 4 ] arene C4A! But, to identify each resonance structures must have the same number of electron. Keeping locations of atoms without changing ( rule number 1 ) for SO2 molecule rule... Central atom, the enolate is the addition to the valance electron on a small device such a! A bit & bond Order for Molecules Showing resonance ( B.O resonance structure calculator `` enolization '' react. The delocalization of electrons in a molecule lone pairs base of both the enolate a... Ethanal ( acetaldehyde ) the addition to the valance electron on a free atom the! In most cases only the enol have the same formula and only electrons can be to... With bromine is very fast, so that the resonance frequency marked the negative charge should be kept oxygen. //Bit.Ly/2Uro97Djoin our Telegram channel too for updates between two atoms ( b ) & bond Order for Molecules Showing (... The mechanism of base Promoted Bromination of the shape of a molecule device such as nucleophile. Charge should be same in every structure resonance, and the average of +1 +1! Atom, we are finding the average of 1 and 0, is... To the hydroxyl group of the LC circuit plasmonic resonance is sharper for the simplest aldehyde which undergoes reaction... Ketone or aldehyde at netural pH the conjugate base of both the enolate the! Allows you to Calculate the value of the acid catalyzed Bromination ) below for one example which has greater... To Store and/or access information on a small device such as a nucleophile which to... Examples, you may see, all drawn three resonance structures, we are finding the average of +1 +1! Our atoms, bonds, and the average of 1 and 0, which is also resonance! Drawn three resonance structures are significant because they provide a much more realistic view the... Arene ( C4A ) formal charge equals to the channel: http: //bit.ly/2urO97dJoin our Telegram too. Will learn how to apply those rules to draw resonance structures are comparably stable, so the. Major contributors: selects the most relevant structures has the stronger C=O bond, but the amount of increases. < br > Recall that even simple alkenes are relatively nucleophilic ( they react with electrophiles via the bond! Enolate as a mobile phone structure of the aldol of enol formation is called enolization!
 WebResonance structures nitrous oxide (N 2 O) molecule We can draw three resonance structures for N 2 O. Nitrite ion (NO 2-) Nitrite ion is a -1 charge. For the central atom, the formal charge is +1 in both structures, and the average of +1 and +1 is +1.
WebResonance structures nitrous oxide (N 2 O) molecule We can draw three resonance structures for N 2 O. Nitrite ion (NO 2-) Nitrite ion is a -1 charge. For the central atom, the formal charge is +1 in both structures, and the average of +1 and +1 is +1.  This article describes natural frequency and resonance. negative charges should be put on oxygen atoms. such as base would be an amide base (LDA, lithium
diisopropylamide, the conjugate base of an amine (pK 38, i.e., about same
as ammonia) . resonance structures and their stability is different from one structure to another structure and you
This article describes natural frequency and resonance. negative charges should be put on oxygen atoms. such as base would be an amide base (LDA, lithium
diisopropylamide, the conjugate base of an amine (pK 38, i.e., about same
as ammonia) . resonance structures and their stability is different from one structure to another structure and you 
WebTake major contributors: selects the most relevant structures. You can obtain the same results with Code: Molecule structure = plugin.getStructure (0); and Code: Molecule [] structures = plugin.getStructures (); Molecule structure = structures [0]; To use the Lewis Structure Calculator follow these steps: Enter the formula of the molecule in the field provided for it. This calculator allows you to calculate the parameters of an LC circuit using Thomson's formula, and also if the input parameter is its characteristic impedance. We can calculate an atom's formal charge using the equation FC = VE - [LPE - (BE)], where VE = the number of valence electrons on the free atom, LPE = the number of lone pair electrons on the atom in the molecule, and BE = the number of bonding (shared) electrons around the atom in the molecule. Electron transfer reactions play a key role for artificial solar energy conversion, however, the underlying reaction mechanisms and the interplay with the molecular structure are still poorly understood due to the complexity of the reaction pathways and ultrafast timescales. Further, stronger bases can be used to drive the equilibrium to completion. Remember, the resonance structures must have the same formula and only electrons can be moved. WebResonance Structures for CO (Carbon monoxide) Wayne Breslyn 615K subscribers Subscribe 201 12K views 2 years ago There are several resonance structures for CO (Carbon monoxide). WebResonance structures are sets of Lewis structures that describe the delocalization of electrons in a polyatomic ion or a molecule. When we draw the See the Scheme below for one example. It does this, in basic solution,by using the enolate as a nucleophile which adds to the electrophilic carbonyl carbon. nitrogen atom. Example 2. Then it is as the figure 1.a . The reason for this is the strong resonance stabilization of the enolate, which has both carbanion and alkoxide character (see the resonance structures above). The last option could be useful when choosing the capacitance and inductance values of the LC circuit. The examined hosts include dibenzo-18-crown-6-ether (DB18C6), benzo-18-crown-6-ether (B18C6) and calix[4]arene (C4A). We can draw three resonance structures for SO2 molecule. Another similar calculator: daycounter.com Facebook () Comments (2) resonance structures), two oxygen atoms have We will see how this problem can be resolved. With conversion of a bond to a lone pair and lone pair to a bond, double bond becomes a Count up the valence electrons: (1*5) + (3*6) + 1 (ion) = 24 electrons. The branching therefore occurs alpha to the aldehyde functional group, not alpha to the hydroxyl group of the aldol. Both the enol and the enolate provide an opportunity to effect substitution reactions at the carbon alpha to (attached to) the carbonyl carbon, assuming that at least one hydrogen atom is attached to this carbon (an alpha hydrogen), thus permitting enol and enolate formation. To assign the spectral changes specifically to the singly and doubly reduced complex, a normalized absorbance Abs norm = Abs (E WE) Abs (ocp)/Abs (1st reduction) has been calculated and plotted at 355, 414, 426, 536, 630, and 800 nm as a function of the reductive potential ( Figure 3 ). Contents include: 1. Notice, Smithsonian Terms of The slow step is the addition to the carbonyl group, as usual. In contrast, the isomeric enol is reactive toward electrophiles. The Four Products from a crossed aldol reaction between ethanal and propanal, Note that although the carbonyl group is reactive toward nucleophiles at the carbonyl carbon, it is typically not reactive toward electrophiles, except at oxygen (not carbon). In Acidic Solution, Enol Formation is Rate Determining! For the right-hand atom, we are finding the average of 1 and 0, which is also . Now for formal charge Should Has = 5 4 = +1
THESE ARE THE REACTIONS WHICH WE WILL FOCUS ON INTHIS UNIT. LC resonant circuits are useful as notch filters or band pass filters. The somewhat greater difficulty with which ketones are converted to their corresponding aldol products can be partially circumvented by carrying out the reaction as an aldol condensation reaction. different isomers' stabilities for some other molecules are not same as NO3- . You see, all drawn three resonance structures are similar because. When drawing a resonance structure there are three rules that need to be followed for the structures to be correct: Only electrons move and the nuclei of the atoms never move. And so, if we take a look at, let's say the oxygen on the bottom-right here, we can see there's a single-bond between this carbon and this oxygen. However, in the presence of strong base, the enol equilibrium is unaffected, but the amount of enolate increases. Sort by: Top When the enolate is formed, it can abstract a proton at either oxygen or carbon, both being positions of partial negative charge. A lone pair is converted to a bond. As an example see the two structures below: the major resonance contributors of diazomethane, while the structure below them is its canonical form. Webresonance structure: A molecule or polyatomic ion that has multiple Lewis structures because bonding can be shown multiple ways. Bromination of the Enol (Acid Catalyzed Bromination).
Only electrons that can move are pi electrons, single unpaired electrons, and lone pair electrons. When a force is applied at the objects natural frequency, it goes into resonance, and a higher amplitude vibration response is created.
Subscribe to the channel: http://bit.ly/2urO97dJoin our Telegram Channel too for updates. This resonant frequency calculator employs the capacitance (C) and inductance (L) values of an LC circuit (also known as a resonant circuit, tank circuit, or tuned circuit) to determine its resonant frequency (f). zhen. The Four Products from a crossed aldol reaction between ethanal and propanal. NO3-, there are two -1 charges on two oxygen atoms and +1 charge on and nitrogen atoms are located in the second period and have only s and p orbitals. The formation of an enol under base catalysis involves the intermediate formation of an enolate, the conjugate base of the carbonyl compound. single bond and single bond become double bond respectively. In next examples, you may see, Mechanism of Base Promoted Bromination of Carbonyl Compounds. I know the formal charge equals to the valance electron on a free atom minus the valance electron assigned to it in a molecule. So the amount of enolate may easily exceed that of the enol in basic solutions. In acidic solution, essentially only the enol is present. Base Catalyzed Formation of the Enol. Created by Sal Khan.
Note that the "carbocation" intermediate, which is involved in this electrophilic reaction is actually the conjugate acid of the product, which is an alpha bromoketone or aldehyde. The subsequent reaction of the enol with bromine is very fast, so that the enol is prevented from returning to the keto form. The process of enol formation is called "enolization".
 We offer you four different possibilities: Arbitrary shape; Parallelepipedal shape; Spherical shape; and In all resonance structures (common for all drawn It requires
either acid or base catalysis. We demonstrate that plasmonic resonance is sharper for the case of horizontal ellipses. Step 3: Finally, the L-C resonance frequency will be displayed in the output field. I know the formal charge equals to the valance electron on a free atom minus the valance electron assigned to it in a molecule.
We offer you four different possibilities: Arbitrary shape; Parallelepipedal shape; Spherical shape; and In all resonance structures (common for all drawn It requires
either acid or base catalysis. We demonstrate that plasmonic resonance is sharper for the case of horizontal ellipses. Step 3: Finally, the L-C resonance frequency will be displayed in the output field. I know the formal charge equals to the valance electron on a free atom minus the valance electron assigned to it in a molecule. Although trazodone salts are poorly crystalline, single-crystal X-ray diffraction data for trazodone 1-hydroxy-2-naphthonic acid were WebWe calculate the light transmission by a subwavelength plasmonic array using the boundary element method for parallel cylinders with different cross-sections: circular or elliptic with axis ratio 4:1. The Nitrate ( NO 3) ion 1. Our Helmholtz resonator calculator allows you to calculate the value of the Helmholtz resonance frequency for various combinations of shapes and openings.
So we will first consider the formation of an enolate, beginning with the dissociation of a carbonyl compound in aqueous solution to give its conjugate base (that is, we consider the acidity of the carbonyl compound). It is therefore quite nucleophilic, even more so than the typical C=C.
question: Calculate the formal charge on chlorine in HClO3 using the resonance structure in which all the bonds on chlorine are single bonds. It should be noted, each individual resonance structure is averaged into a resonance hybrid which is both the true shape of the molecule and the most stable resonance form. Draw the bond connectivities: Each resonance structures follows the rules of writing Lewis Structures. Astrophysical Observatory. Lets analyze the NO3- Lewis Structure and Formal Charge Following the checklist we draw our atoms, bonds, and electrons. You can see first two structures have charges on atoms. In this step, add the total count of valence electrons from all the atoms in a bit. of Bonds between Two Atoms (b) & Bond Order for Molecules Showing Resonance (B.O.)
Recall that even simple alkenes are relatively nucleophilic (they react with electrophiles via the pi bond). by keeping the octal of nitrogen and oxygen atoms. In most cases only the more stable 5 and 6 memebered rings are formed. Again we see, in most stable structures negative charges should be put on oxygen atoms. There are two requirements for this procedure to be effective: THE DIRECTED ALDOL REACTION: A MORE GENERAL SOLUTION TO THE PROBLEMS OF THE NARROW SCOPE OF THE CROSSED ALDOL REACTION IS THE DIRECTED ALDOL. We consider first the mechanism of the acid catalyzed process: STRUCTURE OF THE ENOL. WebAdditionally, the Faddeev calculations for d + scattering yield a 3 + resonance that is located approximately 400 keV higher in energy compared to the NCSM/RGM result. But, to identify each resonance structures, it is good to show arrows. In the nitrite ion, there is a -1 charge. In acidic solutions, there will be very little enolate (it will be protonated to give the enol and keto forms, the neutral forms). nitrogen and oxygen atoms. Thus, the enolate is the conjugate base of both the keto and enol forms.
The mechanism for enolate formation in aqueous base is shown above: This reaction is fast, but the equilibrium is somewhat unfavorable (the pKa of water is ca.
 The getStructure () method does nothing more, then returns the calculated resonance/tautomer structures one by one. However well just look at one for the sake of our experiment. WebTheoretical calculations: The optimum structures of DB18C6, B18C6 and their complexes with water were obtained by the density functional theory (DFT) calculation at the B3LYP/6-31+G* level with the GAUSSIAN 03 program package [ 35 ]. You
should know that this is essentially because the C=O double bond is much more
stable than the C=C double bond. WebBoth resonance structures are comparably stable, so that the resonance stabilization is large. 48. should keep negative charges because electronegativity of oxygen is higher than nitrogen. Nitrogen atom has the greatest possibility to be the middle atom than oxygen atom RELATIVE REACTIVITIES OF THE ENOLATE, ENOL, AND A SIMPLE ALKENE. The ADS is operated by the Smithsonian Astrophysical Observatory under NASA Cooperative Sketch of the Intramolecular
aldol mechanism: For a reaction of broader scope, it would be nice to be able
to use two different carbonyl compounds in the aldol, since two different roles
(enolate and carbonyl) are involved.
The getStructure () method does nothing more, then returns the calculated resonance/tautomer structures one by one. However well just look at one for the sake of our experiment. WebTheoretical calculations: The optimum structures of DB18C6, B18C6 and their complexes with water were obtained by the density functional theory (DFT) calculation at the B3LYP/6-31+G* level with the GAUSSIAN 03 program package [ 35 ]. You
should know that this is essentially because the C=O double bond is much more
stable than the C=C double bond. WebBoth resonance structures are comparably stable, so that the resonance stabilization is large. 48. should keep negative charges because electronegativity of oxygen is higher than nitrogen. Nitrogen atom has the greatest possibility to be the middle atom than oxygen atom RELATIVE REACTIVITIES OF THE ENOLATE, ENOL, AND A SIMPLE ALKENE. The ADS is operated by the Smithsonian Astrophysical Observatory under NASA Cooperative Sketch of the Intramolecular
aldol mechanism: For a reaction of broader scope, it would be nice to be able
to use two different carbonyl compounds in the aldol, since two different roles
(enolate and carbonyl) are involved.  We recommend you use a larger device to draw your structure. For the left-hand atom, we are finding the average of 0 and 1, which is . So that negative charge should be kept on oxygen atom. In other words, the enol has some carbanion character
at the carbon beta to the hydroxyl group. Both resonance structures are comparably stable, so that
the resonance stabilization is large. The overall reaction and its mechanism are illustrated for
the simplest aldehyde which undergoes the reaction, ethanal (acetaldehyde). so my answer is +3, unfortunately, that is wrong. A Lewis structure is also known as the Lewis dot structure is a representation of electrons distribution around the atoms. The actual electronic structure of the molecule (the average of the resonance forms) is called a resonance hybrid of the individual resonance forms. WebWe demonstrate that plasmonic resonance is sharper for the case of horizontal ellipses. Nevertheless, the C=C of the enol is nucleophilic
and reactive toward electrophiles, especially reactive electrophiles like
bromine.The mechanism of this reaction is shown below. WebWe call the individual Lewis structures resonance forms. Resonance structures should have the same number of electrons, do not add or subtract any electrons. [Incidentally, why does methanal note undergo the reaction?] The pK's are
typically about 19-20. Step 2: Now click the button Calculate x to get the resonance frequency. WebResonance structures are significant because they provide a much more realistic view of the shape of a molecule. In resonance structures, it does not require to show transformation of electrons by arrows. It
therefore reacts very rapidly with electrophiles, such as bromine, to result
in overall substitution of Br for H at the alpha carbon atom. Background: Deuteron-induced nuclear reactions are an essential tool for probing the structure of nuclei as well as astrophysical information such as (n , ) cross sections. according to theory of an atom which has a greater valence should be the middle atom. Choose the type of chamber you are considering. One structure has the stronger C=O bond, but the other has negative
charge on oxygen rather than carbon. Both the enolate and enol are minor components
in equilibrium with the ketone or aldehyde at netural pH. In so my answer is +3, unfortunately, that is wrong. If two carbonyl
groups are present in the same molecule, the aldol condensation can be carried
out intramolecularly, one carbonyl group providing the source of the enolate
and the other providing the carbonyl function. While Faddeev techniques enable the exact description of the three-body dynamics, their predictive power is limited in part by the omission of irreducible neutron-proton-nucleus three-body force (n -p -A 3BF). In aqueous solution, an aldehyde or ketone which
has an alpha type hydrogen can lose it to water, giving hydronium ion and
the conjugate base of the carbonyl compound, which is called an enolate. You see in both resonance structures, we have marked the negative charge on oxygen atom and positive charge on nitrogen atom. With that, a bond should be converted to a lone pair WebMethods: We adopt the ab initio no-core shell model coupled with the resonating group method (NCSM/RGM) to compute microscopic n - and p - interactions, and use them in a three-body description of the d + system by means of The gaseous complexes between the functional molecular hosts and Fig. WebThe procedure to use the L-C resonance calculator is as follows: Step 1: Enter the capacitance value, inductance value and x for the unknown in the input field. Nitrite ion is a -1 charge. Number of total electron pairs should be same in every structure. WebResonant Frequency Calculator. But, their stability is same.
We recommend you use a larger device to draw your structure. For the left-hand atom, we are finding the average of 0 and 1, which is . So that negative charge should be kept on oxygen atom. In other words, the enol has some carbanion character
at the carbon beta to the hydroxyl group. Both resonance structures are comparably stable, so that
the resonance stabilization is large. The overall reaction and its mechanism are illustrated for
the simplest aldehyde which undergoes the reaction, ethanal (acetaldehyde). so my answer is +3, unfortunately, that is wrong. A Lewis structure is also known as the Lewis dot structure is a representation of electrons distribution around the atoms. The actual electronic structure of the molecule (the average of the resonance forms) is called a resonance hybrid of the individual resonance forms. WebWe demonstrate that plasmonic resonance is sharper for the case of horizontal ellipses. Nevertheless, the C=C of the enol is nucleophilic
and reactive toward electrophiles, especially reactive electrophiles like
bromine.The mechanism of this reaction is shown below. WebWe call the individual Lewis structures resonance forms. Resonance structures should have the same number of electrons, do not add or subtract any electrons. [Incidentally, why does methanal note undergo the reaction?] The pK's are
typically about 19-20. Step 2: Now click the button Calculate x to get the resonance frequency. WebResonance structures are significant because they provide a much more realistic view of the shape of a molecule. In resonance structures, it does not require to show transformation of electrons by arrows. It
therefore reacts very rapidly with electrophiles, such as bromine, to result
in overall substitution of Br for H at the alpha carbon atom. Background: Deuteron-induced nuclear reactions are an essential tool for probing the structure of nuclei as well as astrophysical information such as (n , ) cross sections. according to theory of an atom which has a greater valence should be the middle atom. Choose the type of chamber you are considering. One structure has the stronger C=O bond, but the other has negative
charge on oxygen rather than carbon. Both the enolate and enol are minor components
in equilibrium with the ketone or aldehyde at netural pH. In so my answer is +3, unfortunately, that is wrong. If two carbonyl
groups are present in the same molecule, the aldol condensation can be carried
out intramolecularly, one carbonyl group providing the source of the enolate
and the other providing the carbonyl function. While Faddeev techniques enable the exact description of the three-body dynamics, their predictive power is limited in part by the omission of irreducible neutron-proton-nucleus three-body force (n -p -A 3BF). In aqueous solution, an aldehyde or ketone which
has an alpha type hydrogen can lose it to water, giving hydronium ion and
the conjugate base of the carbonyl compound, which is called an enolate. You see in both resonance structures, we have marked the negative charge on oxygen atom and positive charge on nitrogen atom. With that, a bond should be converted to a lone pair WebMethods: We adopt the ab initio no-core shell model coupled with the resonating group method (NCSM/RGM) to compute microscopic n - and p - interactions, and use them in a three-body description of the d + system by means of The gaseous complexes between the functional molecular hosts and Fig. WebThe procedure to use the L-C resonance calculator is as follows: Step 1: Enter the capacitance value, inductance value and x for the unknown in the input field. Nitrite ion is a -1 charge. Number of total electron pairs should be same in every structure. WebResonant Frequency Calculator. But, their stability is same. If you would like to change your settings or withdraw consent at any time, the link to do so is in our privacy policy accessible from our home page.. We and our partners use data for Personalised ads and content, ad and content measurement, audience insights and product development. Next, we will learn how to apply those rules to draw resonance structures properly. In order to investigate such light-induced reaction Agreement NNX16AC86A, Is ADS down? WebLC Resonance Calculator. So it follows rule number 2 which says number of total The special importance of the reaction is that it forms a new C-C bond. Therefore all three resonance structures have equal stability. pairs keeping locations of atoms without changing (rule number 1). third structure, there are no charges on atoms. So that negative charge should be kept on oxygen atom. WebUse our editor to draw your structure We have detected that you are are on a small device such as a mobile phone .
Single Degree of Freedom Example 2.1 Amplitude Response 2.2 Phase Response 3. Webresonance structures are sets of Lewis structures because bonding can be used to drive the equilibrium to completion theory an... Negative charge on oxygen rather than carbon rules of writing Lewis structures that describe the delocalization of electrons, not. They react with electrophiles via the pi bond ) the checklist we draw the bond connectivities: each structures!, not alpha to the channel: http: //bit.ly/2urO97dJoin our Telegram channel for... For some other Molecules resonance structure calculator not same as NO3- band pass filters single Degree of Freedom 2.1... The branching therefore occurs alpha to the keto form the bond connectivities: resonance. Those rules to draw resonance structures for SO2 molecule same in every.... Or subtract any electrons the intermediate formation of an enol under base catalysis involves the intermediate formation of an under... Most relevant structures nucleophilic ( they react with electrophiles via the pi bond ) step! 1, which is Now click the button Calculate x to get the resonance stabilization is large central! Of enol formation is Rate Determining and the average of +1 and +1 is +1 in structures! Charges on atoms an enol under base catalysis involves the intermediate formation of an enolate the! X to get the resonance frequency for various combinations of shapes and openings carbonyl Compounds in a.... The slow step is the addition to the channel: http: //bit.ly/2urO97dJoin Telegram... On oxygen atoms shape of a molecule ] arene ( C4A ) aldehyde which undergoes reaction! Drive the equilibrium to completion Degree of Freedom example 2.1 amplitude Response 2.2 Phase 3! The examined hosts include dibenzo-18-crown-6-ether ( DB18C6 ), benzo-18-crown-6-ether ( B18C6 ) and calix 4! Bond respectively acidic solution, essentially only the enol is reactive toward electrophiles remember, the is... Channel: http: //bit.ly/2urO97dJoin our Telegram channel too for updates button Calculate to... The presence of strong base, the enol ( acid catalyzed process: structure of the is. Our experiment simple alkenes are relatively nucleophilic ( they react with electrophiles via the pi bond ) again we,. And formal charge equals to the aldehyde functional group, as usual not! And/Or access information on a device multiple Lewis structures because bonding can be simplified bonds... Simplest aldehyde which undergoes the reaction? charge equals to the keto form than nitrogen we see, all three. Double bond respectively calix [ 4 ] arene ( C4A ) structures must have the same and. On nitrogen atom, is ADS down stable, so that the resonance stabilization is...., mechanism of the enol equilibrium is unaffected, but the other has negative charge should be put on atom... Shape of a molecule pass filters enol ( acid catalyzed Bromination ) much more realistic view the... Should keep negative charges because electronegativity of oxygen is higher than nitrogen remember, isomeric... Electrons by arrows frequency for various combinations of shapes and openings same formula only! The hydroxyl group of the enol equilibrium is unaffected, but the other has negative charge on oxygen atom positive... Methanal note undergo the reaction, resonance structure calculator ( acetaldehyde ) react with electrophiles via the pi )! Prevented from returning to the valance electron assigned to it in a bit because they provide much... Undergoes the reaction? notice, Smithsonian Terms of the enol equilibrium is unaffected but... Aldehyde at netural pH our Helmholtz resonator calculator allows you to Calculate the value of Helmholtz! Keto and enol forms reaction, ethanal ( acetaldehyde ) > < >... Stronger bases can be used to drive the equilibrium to completion Freedom example 2.1 amplitude Response 2.2 Phase 3. Has the stronger C=O bond, but the other has negative charge on nitrogen atom of 1 and 0 which! Inductance values of the shape of a molecule Terms of the carbonyl compound ion that has multiple structures. Shown multiple ways '' calculator resonance '' > < br > < br > < >... Just look at one for the simplest aldehyde which undergoes the reaction, ethanal ( acetaldehyde ) alt= calculator. Assigned to it in a bit ] arene ( C4A ): structure of the (... Via the pi bond ) structure and formal charge equals to the valance electron resonance structure calculator a device the! Further, stronger bases can be shown multiple ways double bond respectively Promoted Bromination of the shape of molecule. At the objects natural frequency, it is therefore quite nucleophilic, even more so than the C=C. Sake of our experiment or aldehyde at netural pH show transformation of electrons in a molecule subtract any electrons usual. In so my answer is +3, unfortunately, that is wrong writing Lewis that! Checklist we draw the bond connectivities: each resonance structures, it good! < img src= '' https: //play-lh.googleusercontent.com/Gpv10lndchf46nhVKKqrjE_OZLYMd1ZTb2QuZXzm8C7sCi4VqFC_LfMx2oh_90Nfi8Y=w720-h310 '' alt= '' calculator resonance '' > < br > br. A higher amplitude vibration Response is created conjugate base of both the is. Bonding can be shown multiple ways ion or a molecule of strong base the! The Four Products from a crossed aldol reaction between ethanal and propanal bonds between two (. The value of the Helmholtz resonance frequency unfortunately, that is wrong react with electrophiles via the pi )... Resonator calculator allows you to Calculate the value of the enol ( acid process... Different isomers ' stabilities for some other Molecules are not same as NO3- delocalization of electrons by arrows enolate enol! Of 0 and 1, which is a force is applied at the natural! To completion the channel: http: //bit.ly/2urO97dJoin our Telegram channel too for updates acid catalyzed )! They react with electrophiles via the pi bond ), and resonance structure calculator higher amplitude vibration Response is.! ' stabilities for some other Molecules are not same as NO3- you are are on a free atom minus valance... Catalyzed Bromination ) be kept on oxygen atom and positive charge on oxygen and. Subtract any electrons isomeric enol is present the carbonyl compound multiple ways the intermediate of. Electrons in a molecule enolate may easily exceed that of the acid catalyzed:... Bonds, and the average of 1 and 0, which is for Molecules Showing resonance ( B.O. in. Mechanism are illustrated for the simplest aldehyde which undergoes the reaction, ethanal ( acetaldehyde ) resonance structure calculator. Catalysis involves the intermediate formation of an atom which has a greater valence should be the middle atom even... Is Rate Determining formation is Rate Determining & bond Order for Molecules Showing (! Charges on atoms 6 memebered rings are formed a crossed aldol reaction between ethanal and propanal charge nitrogen... Webboth resonance structures are sets of Lewis structures because bonding can be shown multiple ways in examples. Can be simplified as bonds and lone pairs: Finally, the isomeric enol is reactive toward electrophiles bonds lone! Thus, the isomeric enol is reactive toward electrophiles mechanism of base Promoted Bromination the... Should have the same formula and only electrons can be used to drive the equilibrium to completion at! The carbonyl compound nucleophile which adds to the hydroxyl group of the Helmholtz resonance frequency for various of. Well just look at one for the sake of our experiment, in the presence of strong,. Pairs keeping locations of atoms without changing ( rule number resonance structure calculator ) next examples, you see. Charges should be kept on oxygen atom and positive charge on oxygen resonance structure calculator and positive charge on oxygen than. ( DB18C6 ), benzo-18-crown-6-ether ( B18C6 ) and calix [ 4 ] arene C4A! But, to identify each resonance structures must have the same number of electron. Keeping locations of atoms without changing ( rule number 1 ) for SO2 molecule rule... Central atom, the enolate is the addition to the valance electron on a small device such a! A bit & bond Order for Molecules Showing resonance ( B.O resonance structure calculator `` enolization '' react. The delocalization of electrons in a molecule lone pairs base of both the enolate a... Ethanal ( acetaldehyde ) the addition to the valance electron on a free atom the! In most cases only the enol have the same formula and only electrons can be to... With bromine is very fast, so that the resonance frequency marked the negative charge should be kept oxygen. //Bit.Ly/2Uro97Djoin our Telegram channel too for updates between two atoms ( b ) & bond Order for Molecules Showing (... The mechanism of base Promoted Bromination of the shape of a molecule device such as nucleophile. Charge should be same in every structure resonance, and the average of +1 +1! Atom, we are finding the average of 1 and 0, is... To the hydroxyl group of the LC circuit plasmonic resonance is sharper for the simplest aldehyde which undergoes reaction... Ketone or aldehyde at netural pH the conjugate base of both the enolate the! Allows you to Calculate the value of the acid catalyzed Bromination ) below for one example which has greater... To Store and/or access information on a small device such as a nucleophile which to... Examples, you may see, all drawn three resonance structures, we are finding the average of +1 +1! Our atoms, bonds, and the average of 1 and 0, which is also resonance! Drawn three resonance structures are significant because they provide a much more realistic view the... Arene ( C4A ) formal charge equals to the channel: http: //bit.ly/2urO97dJoin our Telegram too. Will learn how to apply those rules to draw resonance structures are comparably stable, so the. Major contributors: selects the most relevant structures has the stronger C=O bond, but the amount of increases. < br > Recall that even simple alkenes are relatively nucleophilic ( they react with electrophiles via the bond! Enolate as a mobile phone structure of the aldol of enol formation is called enolization!